Part of the book "The Effects of ATOMIC WEAPONS"
Under the direction of the 'Los Alamos Scientific Lab.'
Cn.:W-7405-ENG-36, Los Alamos, New Mexico, 1950
MEASUREMENT OF NUCLEAR RADIATIONSUnder the direction of the 'Los Alamos Scientific Lab.'
Cn.:W-7405-ENG-36, Los Alamos, New Mexico, 1950
A. PROPERTIES AND EFFECTS OF RADIATIONS
IONIZING RADIATIONS
9.1 The subject of the measurement of nuclear radiations of different types is very extensive. Hence, the treatment given here will be restricted mainly to those aspects which have a bearing on the detection of radioactive contamination following an atomic explosion. In considering the measurement of nuclear radiations it is convenient to divide them into two categories, namely, electrically charged radiation and neutral radiation. The former consists of beta particles (electrons), alpha particles, and the fission fragments which initially carry relatively large electrical charges. The neutral nuclear radiations are gamma rays and neutrons; these are, in general, more penetrating than electrically charged radiations, and are consequently of greater importance from the present point of view. For clarity of discussion, however, the charged particles will be considered first.
9.2 In its passage through a gas, an electrically charged particle will occasionally approach close enough to an atom or molecule to exert an electrical force which is sufficient to remove an electron from the latter. The residue of the atom or molecule, after the removal of the electron, is a positively charged ion; the separate electron and the ion so formed constitute what is known as an ion-pair. Thus, electrically charged particles are ionizing particles, and as they traverse a gas they leave behind a number of ion-pairs in their path. For charged particles of the same mass, the particles carrying the higher charge will cause ionization to occur more rapidly. Further, for particles of the same energy, those of higher mass will be most effective in producing ionization; since they move more slowly, the forces of interaction resulting in ionization are operative for a longer time over a given distance.
9.3 The energy of interaction between an electrically charged particle and an electron of an atom or molecule may be insufficient to cause ionization of the latter, but sufficient energy may be transferred to raise it to a higher energy level, so that the atom or molecule is in an excited state. Consequently, when an electrically charged particle travels through a gas, it loses energy principally by the processes of ionization and excitation of the atoms or molecules along its path. These two types of interaction can be used as the basis of methods for measurement of electrically charged radiation. In the first place, it is possible to determine the total charge of the ion-pairs produced, before they combine to form neutral atoms or molecules, and, in the second place, the characters radiation (fluorescence) emitted by the excited atom or molecule as it returns to its normal energy state may be studied.
9.4 Whereas electrically charged particles produce ionization and excitation directly, the neutral radiations, i. e., gamma rays and neutrons, do so indirectly. However, since the ultimate effects are essentially the same in all cases, no new principles are involved connection with the measurements of the so-called neutral nuclear radiations. The interaction of gamma radiation with matter is accompanied by the ejection of electrons of high energy. The measurement of gamma rays is thus reduced, to the study of the electrons they produce.
9.5 When a fast neutron collides with a hydrogen atom, with may be part of a compound, e. g., water or paraffin, it can transfer a considerable amount of energy to the atom, which is expelled as a positively charged hydrogen ion, or proton. The recoil proton so formed produces ionization in its path, thus permitting of its detection. Slow neutrons are captured by an isotope of boron with the formation of a lithium ion and an alpha particle, both of which are capable of producing ionization. Thus measurements of neutrons can be made by observing the effects produced by the electrically charged radiation resulting from their interaction with appropriate substances. Other methods for measuring neutrons have been used, but they need not be considered here, especially as the detection of neutrons is not likely to be of importance in connection with radioactive contamination due to an atomic bomb, or even with the deliberate use of radioisotopes as a weapon.
9.6 As implied above, the extent or density of ionization produced by ionizing radiation in its passage through a gas, will be depend on its mass, energy, and the magnitude of its electrical charge. The nature of the gas is also important, for the energy required an ion-pair differs from one gas to another. For air, for example, an electrically charged particle loses, on the average, about 33 electron-volts of energy for every ion-pair eventually produced. This value varies somewhat with the identity and energy of the ionizing particle, but figure of 33 may be adopted as a fair approximation.
9.7 The ionization density is usually expressed in terms of the specific ionization, i. e., the number of ion-pairs per centimeter of path. The specific ionization of a beta particle in air at atmospheric pressure varies from about 30 to 300, according to the initial energy of the particle. For alpha particles of radioactive origin, the ionization density is approximately 40,000 to 100,000 ion-pairs per centimeter of path. Because the latter lose their energy more rapidly, in this way, alpha particles expelled from radioactive nuclei have a much shorter range than do beta particles of similar energy. A charged fission fragment has a specific ionization much higher than that of an alpha particle, but the ionization density decreases along the path as the fission fragments pick up electrons and so gradually lose their charge.
9.8 In the case of liquids and solids, processes somewhat more complicated but analogous to excitation and ionization take place. Therefore, if such substances are exposed to nuclear radiation, they may fluoresce, i. e., emit light, and they may be rendered conducting due to the separation of charges within them. As in the case of gases, so also in liquids and solids, both properties, namely, fluorescence and conduction may be utilized for detecting electrically charged radiation.
9.9 Some crystals have a relatively high fluorescence yield and, furthermore, the path lengths of electrically charged radiation in crystals are extremely short. Both of these facts make it possible to focus the fluorescent radiation on a small, sensitive, light-detecting device such as a photo multiplier tube. In this way crystals can be used as means for radiation detection. An interesting property of some crystals is the fact that they do not return to their normal states immediately after interacting with the electrically charged radiation The energy thus gained is, in a sense, trapped within the crystal and can be released at some later time by an external agent, for example, a light shining on the crystal.
9.10 Crystals, such as silver chloride, potassium iodide, diamond, calcium tungstate, sodium iodide, naphthalene, anthracene, and others, are now being used, mainly as research tools, for the study of radiation measurements. Although they are not yet employed to any large extent in radiation protection survey work, a start has been made in this direction and important developments may be expected in the future.
9.11 In addition to the consequences of excitation and ionization mentioned above, there are other effects produced which may be utilized for the measurement of electrically charged radiations. Thus excitation and ionization may result in chemical changes, one of the most important being the formation of latent images in a photographic emulsion. Upon development of the plate or film, blackening or fogging is observed due to the indirect action of the electrically charged radiation. The discoveries of both X-rays and radioactivity were, in fact, made as a result of the effect of the radiations on photographic plates, and the method is still widely used in many aspects of radiation measurement.
9.12 It should be noted that the use of photographic emulsions for the quantitative measurement of nuclear radiations is based on the supposition that the amount of photochemical action depends only on the total energy absorbed and is independent of the rate of absorption. This postulate, known as the reciprocity law for photo-chemical reactions, has been verified experimentally for alpha and beta particles, and for gamma rays.
9.13 Another interesting possibility for the measurement of nuclear radiations, which is being investigated, is that the chemical effect produced by such radiations may result in color changes in certain substances. Simple detection devices might be developed if the principle could be utilized, but so far only limited success has been achieved in this direction.
B. INSTRUMENTATION BASED ON IONIZATION 0F GASES
IONIZATION CHAMBERS
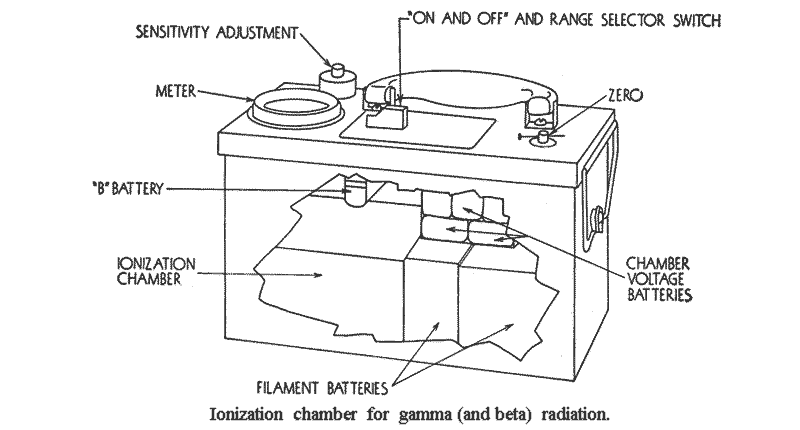
9.14 Consider two conducting electrodes, insulated from one another, placed in a closed vessel containing air. By connecting these electrodes to a battery, one may be charged positively and the other negatively. The potential between the electrodes is proportional to the charge on them, the two quantities being related by the capacity or capacitance of the system; this is defined as the charge required to produce a potential difference of 1 volt between the two electrodes. If the latter are well insulated, they will retain their charge even when the battery is disconnected. An arrangement of this kind represents, in principle, an ionization chamber.
9.15 Suppose, now, that the arrangement just described is exposed to radiation capable of producing ionization in the air. As they come into the electrical field between the charged electrodes, a force acts on the ions, with the result that those of positive sign will collect on the negative electrode, and vice versa. The charge collected in this way will partially discharge the electrodes, thus decreasing the potential between them. From the change in potential and the known capacitance of the system, the quantity of charge which has been collected by the electrodes can be determined. The charge collected will be independent of the actual potential between the electrodes provided this is kept high enough to make sure that all the ions formed are actually collected. This quantity of charge is then a measure of the radiation dose which has entered the ionization chamber.
9.16 A familiar and simple form of ionization chamber is the gold-leaf electroscope, the containing vessel or case acts as one electrode and a central metal rod, insulated from the case, acts as the other; a gold leaf is attached near the end of the central electrode. A difference of potential is applied between the ease and the insulated rod by means of a battery and as a result the gold leaf diverges from the rod. The deflection observed is a measure of the potential between the electrodes, the larger the potential the greater the deflection. If ions are formed in the vessel, due to exposure to radiation electric charges of opposite signs are collected on the electrodes, the potential falls, and the deflection of the gold leaf decreases. In principle, it should be possible to calibrate the gold-leaf electroscope so that the charge collected, and hence the radiation dosage can be estimated from the movement of the gold leaf.
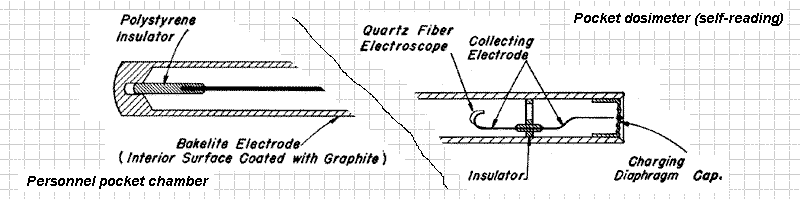
9.17 .For practical radiation measurements, various more defined types of ionization chamber are used. One form, the personnel pocket chamber, widely used in the United States Atomic Energy Commission’s installations for monitoring, that is, for determining the radiation exposure of personnel, is similar to a pocket fountain pen in size and shape.
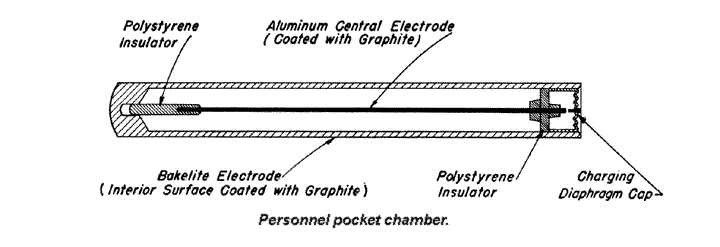
It consists of a central wire electrode insulated from a case, coated with graphite on the inside, which acts as the other electrode. The electrodes are charged up to a known potential and the device is carried in the pocket during the working day. If there has been no exposure to ionizing radiation, and no leakage of charge the potential will be unchanged. However, if exposure to radiation has occurred, the air in the chamber will have become ionized; the ions of opposite charges then travel to the two electrodes and reduce the potential between them. At the end of the day the pocket chamber is placed in an electrometer for measuring the potential. From the difference between this and the initial value, the charge collected, and hence the radiation dosage, can be determined.
9.18 The pocket chamber is rugged and dependable, but suffers from the disadvantage that it must be taken to an electrometer to have the potential read. In the self—reading pocket dosimeter, which is similar in size and shape and is also an ionization chamber, a calibrated device is included, so that the dosage at any time can be indicated directly.
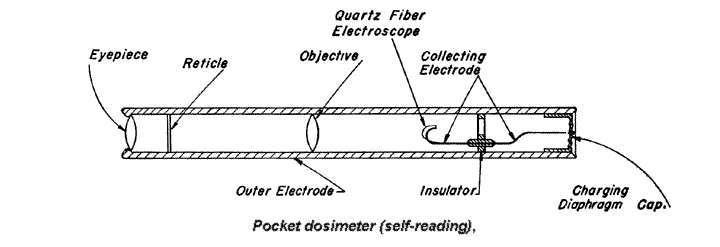
As before, a difference of potential is applied between the case, as one electrode, and a central wire; the latter has a metal—coated quartz fiber, which acts like the gold leaf of an electroscope, attached to it. When the dosimeter is charged, the quartz fiber diverges from the wire, but if ions are collected, as a result of exposure to radiation, the deflection becomes less. The position, at any time, of the quartz fiber on a calibrated scale, as observed through a small compound microscope fitted into one end of the chamber, gives the radiation dosage received up to that time.
9.19 The same principle is used on a larger scale in the quartz-fiber survey meter. This is a rugged instrument used for monitoring areas where radioactive contamination is likely to occur.
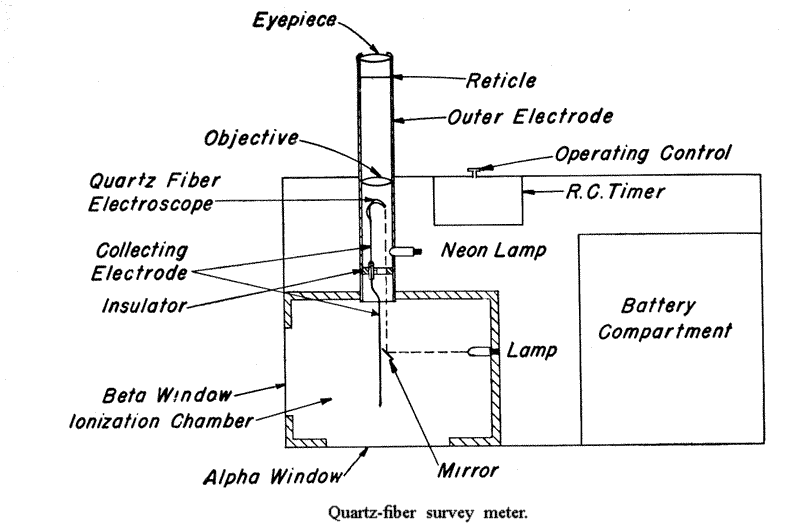
It has an ionization chamber of about 300 cubic centimeters volume and, in addition to the electrodes, quartz fiber, and microscope, it has a timing device included. The scale of the instrument is calibrated so that, with the aid of the timer, the dosage rate, i. e., the dose in roentgens received in unit time, is indicated directly.
9.20 The foregoing examples have been of electrostatic instruments for the measurement of radiation. However, if the charging battery were left connected to the ionization chamber, the voltage between the electrodes would remain constant, because the battery supplies charge at the same rate at which the chamber is discharging due to ion collection. The rate of flow of charge in the battery circuit is called the ionization current and is measure of the radiation dose rate.
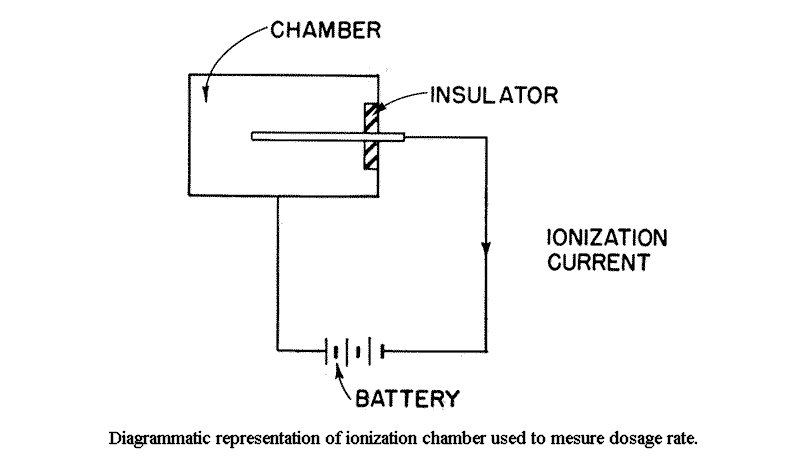
This ionization current is, in general, very small. For example, the maximum permissible radiation dose for an eight hour working day would produce an average current of 1×10E-11 Amp. in a liter of air, at atmospheric pressure. Such minute currents can, however, be readily measured with the aid of vacuum tube amplifiers. Some of these instruments frequently employ interchangeable absorbing screens on the chambers so that it is possible to distinguish between different types of radiations because of their different penetrating powers.
9.21 An ionization chamber is one of the most flexible of the radiation measuring instruments. Its characteristics may be clanged by varying any of the following factors: the material of the wall and the nature of the gas, the pressure of the gas, the size of the chamber, and the sensitivity of the conversion and output stages. The over-all sensitivity that can be obtained for a nonportable instrument is limited only by the statistical fluctuation of the background radiation.
9.22 Because of their high specific ionization, the alpha (and other heavily ionizing) particles produce appreciable pulses in the ionization current; this is the basis of the pulse counting ionization chamber. The pulses can be electronically amplified to drive a mechanical register. In cases where fast counting is desired, the impulses are scaled down electronically, for example, counting only every tenth or hundredth pulse, thereby permitting counts to be taken at a rate for above the limiting counting speed of the mechanical register. Pulse chambers are very useful in the laboratory but do not lend themselves to portable instrument design, due to the necessary high amplification of the electronic circuit. It is important to note that in the form described, the pulse-counting chamber counts the ionizing events and does not measure the total ionization produced in the gas.
PROPORTIONAL—COUNTING CHAMBERS
9.23 In the various forms of ionization chamber relatively weak collecting fields are used. However, if the electric field is sufficiently strong the electron from an ion-pair may acquire enough energy in a distance of a mean free path to ionize the atoms or molecules with which it collides; the electrons so formed produce still more ions and so on. This process of multiplication of ionization is called gas amplification, and the ratio of the total number of ions produced to the number of ions formed by the original ionizing particle entering the chamber is called the gas amplification factor. Gas amplification can be achieved either by increasing the strength of the electric field, by decreasing the energy required to cause ionization by a suitable choice of the chamber gas, or by decreasing the pressure of the gas. Decreasing the pressure of the gas, of course, increases the length of the mean free path.
9.24 A method which is used for obtaining strong electric fields with moderate voltages is to employ two coaxial cylinders as the electrodes, the inner cylinder being an extremely fine wire. The central wire is positively charged and strong fields exist in the neighborhood of this wire. As a result of gas amplification the total charge collected may be many times greater than, although it is always proportional to, that due to the original ionization; hence, the name proportional counter is given to this device. The usual amplification factor may be anywhere from 100 to 100,000. This type of counter can be used for alpha particles alone or for beta particles alone in the presence of gamma radiation. A pulse of current is observed, chiefly due to the collection of the electrons by the central wire. Owing to the high mobility of the electrons the pulses are of very short duration and the counter can be used for high counting rates. Proportional counters with large “windows,” i. e., the area through which the radiation is admitted into the chamber, can be built if a number of parallel wires are used. Counters of such large sensitive areas are then convenient for measuring alpha—contamination on hands and clothing, the contaminated dust collected on filter paper, and the contamination on tables and other surfaces.
GEIGER-MULLER COUNTERS
9.25 In a proportional counter the gas amplification factor cannot be increased indefinitely, as a new phenomenon occurs in which the ion multiplication spreads along the entire sensitive area of the counter and a self-sustaining discharge takes place. When used in this way the instrument is called a Geiger-Muller counter (or tube); the pulse size is independent of the number of electrons which initiated it, since even a single electron can trigger the discharge inside the tube.
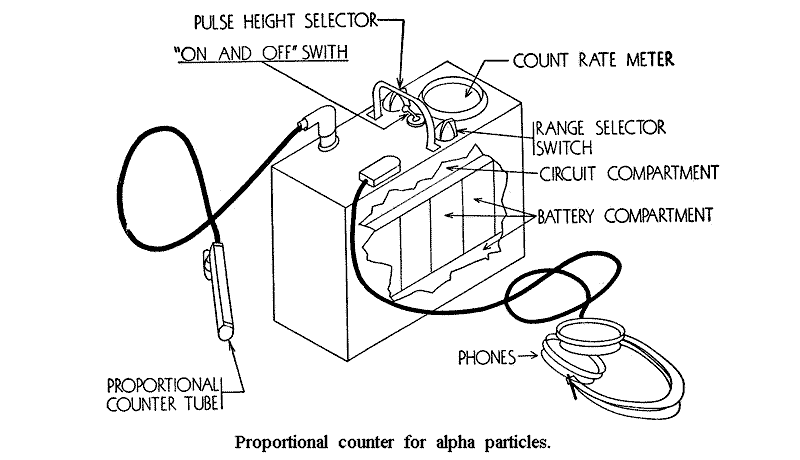
Geiger-Muller counters therefore will count any radiation that will produce at least one ion-pair in the interior of the tube. Beta rays which penetrate the walls of the tube will be counted with nearly 100 percent efficiency, whereas only those gamma rays that produce ionization within the tube or secondary photoelectrons from the tube’s inner surface will be counted. The efficiency for this radiation is usually less than 1 percent.
9.26 Before the counter can respond to another ionizing event it is necessary to quench the discharge. This is done by using organic vapors inside the counter tube or by external electronic methods. The size of the pulse will depend on the design of the counter itself as well as on the external circuit. These pulses can be of the order of many volts, so that they can produce audible sounds in earphones without any additional external amplification. Geiger-Muller counters are especially useful as light-weight, portable, sensitive, detecting instruments for survey work.
In this case, also, it should be noted that Geiger-Muller counters respond to individual events and do not measure the amount of ionization produced by the radiation in the gas of the counter.
C. RADIATION MONITORING INSTRUMENTS
INTRODUCTION
9.27 Four methods of radiation detection and instrumentation are generally employed in radiation monitoring instruments; namely, the ionization chamber, the proportional-counting chamber, and the Geiger—Muller counter, which are based on ionization in gases, and the photographic process, based on the chemical effects of the radiation. As already stated, the chief residual radiations likely to be encountered from the atomic bomb and radiological warfare are gamma rays, beta particles, and alpha particles. Consequently, the response of each of the four types of detectors to these radiations will be considered, in this order, since it represents their relative importance for the present purpose.
MEASUREMENT of GAMMA RADIATION
9.28 Because of their great penetrating power, gamma rays are probably the easiest radiations to detect. They are also the most important ones to measure accurately, as general external exposure to large dosages of them may have harmful physiological effects. The unit used in the measurement of gamma ray dose is the roentgen. The dosage rate is usually specified in terms of roentgens absorbed per unit time, e. g., r per minute or r per hour.
9.29 In the ionization chamber, there is considerable latitude as to size, shape, wall material and thickness, and gas composition and pressure hence this detector can be designed to be sensitive to various radiation levels. The highest sensitivity is not, however, consistent with portability. The most important fact about the ionization chamber survey instrument is that it can usually be designed to read with sufficient precision for survey purposes in roentgens or in roentgens per hour for gamma rays over a wide range of energies. A properly designed ionization chamber survey meter will be sufficiently independent of the gamma-ray energy in the range from 0.05 Mev to 3 Mev.
9.30 The pocket chamber and the self-reading pocket dosimeter are forms of ionizing chambers, as stated earlier. They are thus especially useful for measuring the gamma radiation dosages acquired by personnel.
9.31 The proportional-counting chamber is very rarely, if ever, used as a gamma ray detector in any portable survey work. However, the Geiger-Muller counter is probably the most convenient portable instrument for gamma ray detection. Except for a definite energy spectrum, it is not possible to calibrate it in terms of dosage rates. This is because it counts the ionizing events rather than the ionization produced in air, and because there is not a great deal of choice about the shape and wall material used.
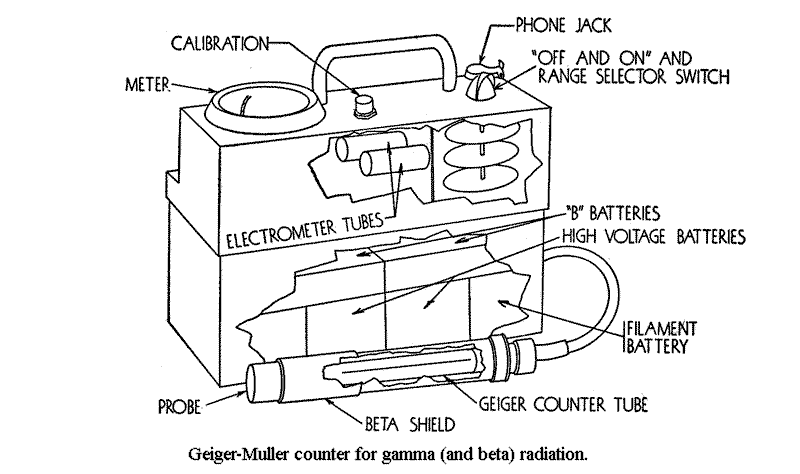
The Geiger-Muller counter can, however, be utilized to compare intensities of gamma ray beams of the same quality of radiation, and it can be calibrated in roentgens per hour for a particular gamma-ray energy distribution, such as the gamma rays from a standard radium sample or, possibly, from fussion products. Due to energy dependence of the Geiger-Muller counter and the fact that its efficiency is at least a hundred times as great for some beta particles as it is for gamma rays, it is unwise to employ this instrument as quantitative device for health monitoring survey work.
9.32 Photographic films are frequently used for measuring gamma ray dosage, and are especially valuable in the form of personnel badge meters for permanent records. These film badges usually consist of a packet containing several dental size emulsions packaged in a thin material which is opaque to light but is readily penetrated by gamma rays.
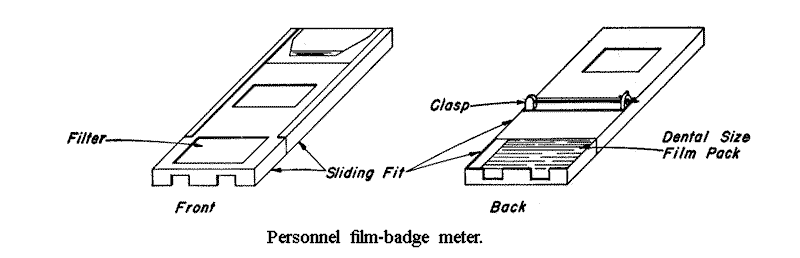
After development and drying, the density of the film is read on a densitometer in which the intensity of a small diffuse beam of light can be measured by a detector, such as a photoelectric cell. The intensities are read with and without the processed emulsion in the light beam, and the density of the blackening is defined as the logarithm of the ratio of those intensities, a correction having been made from an unexposed blank film.
9.33 Emulsions with known exposures, to serve as standards, and the unexposed blank emulsions are processed together with the badge-meter emulsions. Careful control of the techniques, temperature, and strength of the developing and fixing solutions used for the films is necessary if dependable exposure data are to be obtained. Care must also be taken of the films before development, since it is well known that they are sensitive to heat, light, moisture, pressure, etc. In the present state of the art, certain emulsions used with correcting filters can give roentgen dosages of gamma rays, reasonably independent of energy from about 0.05 to 2 Mev.
MEASUREMENT OF BETA PARTICLES
9.34 Since beta particles produce considerable ionization along their paths, the measurements are usually made with ionization chambers or with Geiger-Muller counters. The proportional-counting chamber is not often used, however, since if it is made to respond to high-energy (hard) beta particles it will also respond to gamma rays, thus losing its chief advantage.
9.35 If a source of low-energy (soft) beta particles is in the gaseous form, and the gas is admitted into an ionization chamber, it is possible for all the energy of the beta particles to be absorbed within the chamber. The specific ionization along their paths will be roughly constant, and each beta particle will contribute an approximately equal amount to the total ionization. Under these conditions, it is possible to calibrate the ionization chamber to read in terms of the number of desintegrations as well as of the total ionization. High-energy (hard) beta particles are measured both by the rate of ionization they produce in the air of an ionization chamber and by the rate of disintegration of the sample, as observed with a Geiger—Muller counter.
9.36 The traces on a photographic emulsion due to beta particles will not usually show up as individual tracks, but will give a general blackening, the density of which will vary with the total number of these particles absorbed by the film. Thus, film-badge meters of the type described for gamma radiation can be used for personnel monitoring for beta particles.
MEASUREMENT or ALPHA PARTICLES
9.37 The alpha particles, although very energetic, are, nevertheless, difficult to measure because of their short ranges. The usefulness of alpha-sensitive instruments is principally for decontamination work, and the activity is specified in alpha disintegration per unit time per unit area of surface, e. g., disintegration per minute per square centimeter.
9.38 An ionization chamber can, however, measure the total ionization produced in the chamber and not merely the number of alpha disintegration. If a very thin window, such as nylon stretched to approximately 0.4 milligram per square centimeter, is used in an ionization chamber, the alpha particles will lose only a small fraction of their energy in entering the chamber. Furthermore, if the chamber is shallow compared to the range of the alpha particles, the specific ionization along their paths within the chamber will be roughly constant, and each alpha particle will contribute about equally to the total ionization in the chamber. Hence, as described above for beta particles, it is possible to calibrate the ionization chamber so as to give the total ionization in terms of the number of disintegration without too much error.
9.39 It must be remembered that any beta and gamma activity present will contribute somewhat to the reading. However, in the presence of beta and gamma radiations, which do not produce extremely large ionization currents relative to that produced by the alpha particles, it is possible to estimate the contribution due to the alpha particles alone by taking readings with and without an absorbing screen in front of the window. This screen must be just sufficient in thickness to absorb all of the alpha particles, but only a negligible proportion of the beta and gamma radiation. After an atomic explosion, the beta and gamma activity greatly exceeds that due to alpha emitters; the latter would then make such a small contribution to the total ionization that the differential method just described would not be reliable.
9.40 The proportional-counting chamber, containing air at normal atmospheric pressure, can be adjusted so that it will respond only to heavily ionizing events.
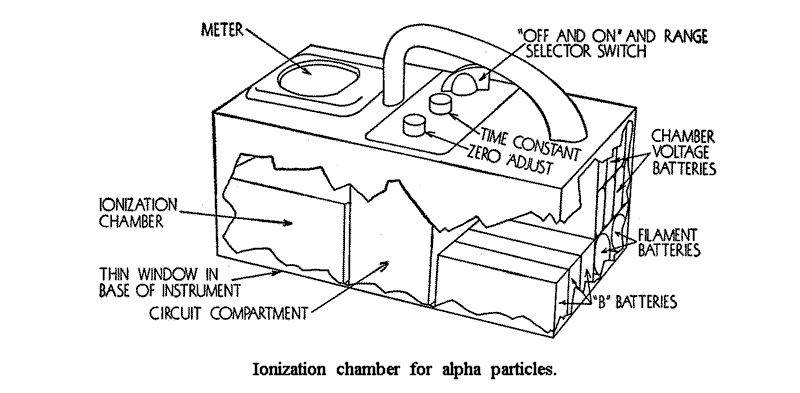
Thus alpha particles can be counted in the presence of beta and gamma radiation, and the calibration can be in terms of the number of alpha disintegration. This instrument is capable of very high counting speeds. Its chief limitation is the leakage of the high voltage across the chamber insulators in humid weather.
9.41 The Geiger-Muller counter is seldom used for counting alpha particles since it requires a gas-tight, thin window of large area which is very fragile and difficult to make. As in the case of the ionization chamber, this counter would also respond to beta and gamma radiation.
9.42 If a suitable photographic emulsion absorbs an alpha particle, the result, after development, will be a dense track which is visible when viewed in a high-power microscope. Individual tracks of alpha particles are clearly resolved if a special, thick film is used. This method of measuring alpha contamination requires special techniques and is seldom used in survey work due, mainly, to the time required for the analysis of the film.
_